
Restore Medical wins FDA breakthrough nod for heart failure treatment device
Restore Medical announced today that it received FDA breakthrough device designation for its ContraBand heart failure treatment device.

Restore Medical announced today that it received FDA breakthrough device designation for its ContraBand heart failure treatment device.

Imperative Care announced today that the FDA granted 510(k) clearance for its Zoom 6F insert catheters for ischemic stroke procedures.

Hyperfine (Nasdaq:HYPR) announced today that the FDA cleared its ninth-generation AI-powered Swoop system software.

Smith+Nephew (NYSE: SNN)+
announced today that it received FDA 510(k) clearance for its new Catalystem primary hip system.

Philips (NYSE: PHG)+
announced today that it received FDA clearance for its latest pathology solution, the IntelliSite Pathology Solution 5.1.
Oticon Medical announced today that the FDA granted clearance for its Sentio transcutaneous bone conduction hearing system.

REMSleep Holdings Inc has received FDA 510(K) clearance for its new DeltaWave nasal pillow CPAP interface mask. The DeltaWave is designed to enhance comfort and maintain natural breathing patterns, aiming to address common issues that lead to therapy discontinuation

ImmersiveTouch announced today that it received FDA 510(k) clearance for its ImmersiveAR augmented reality technology platform.
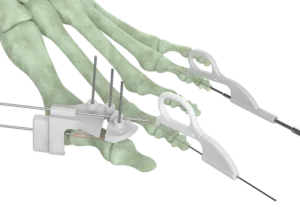
CAMP HILL, Pa.–(BUSINESS WIRE)–Forma Medical Inc., a leading innovator in medical device technologies, is thrilled to announce the FDA 510(k) clearance for its groundbreaking OptimalMTP® Plating System. This regulatory milestone marks a significant leap forward in MIS (Minimally Invasive Surgery), ushering in a new era of precision and efficiency dedicated to foot and ankle surgery, one of the fastest growing segments in the medical device space.

Orthofix (Nasdaq: OFIX) today announced it received FDA 510(k) clearance for the Fitbone Transport and Lengthening System and reported the first implant of the device.