MedTech News
.................... by Andrew Celentano

Explant Express® Receives FDA 510(k) Clearance for Breast Implant Suction Retrieval Device
BRECKSVILLE, Ohio, Feb. 12, 2026 /PRNewswire/ — Applied Medical Technology, Inc. (AMT) today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for Explant Express®, a breast implant removal device designed to support efficient, controlled explantation of ruptured silicone breast implants.

Non-contractile heart cells help sustain persistent atrial fibrillation, study reveals
A multidisciplinary study led by the Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) and published in Circulation Research provides a new perspective on why this arrhythmia can persist long-term, highlighting the key role of non-contractile cardiac cells.
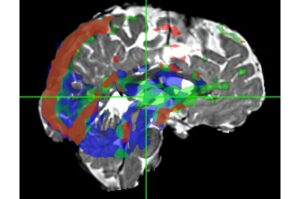
New MRI technique maps fluid velocity distribution in the brain
A new MRI technique called Velocity Spectrum Imaging can map fluid movement in the human brain within a 3D pixel.
3D MRI technique guides precision treatment of kids’ heart conditions
With a new MRI technique that shows both heart tissue and blood flow simultaneously, physicians can see where heart defects occur and precisely plan to repair them, according to new research.
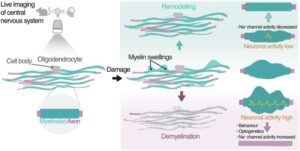
Specialized microscopy enables researchers to visualize the dynamics of myelin swellings
An international research team has gained new insights into the dynamics of myelin swellings in the brain.
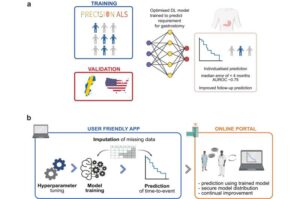
AI model may predict when MND patients need feeding tubes within months
A new AI tool that accurately predicts the need for a feeding tube could transform patient care and improve quality of life for people living with Motor Neuron Disease (MND).
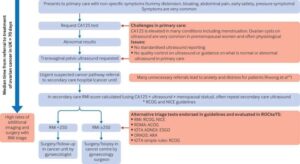
Best diagnostic test for ovarian cancer in premenopausal women identified
The IOTA ADNEX ultrasound tests picked up 9 out of 10 women with cancer.

New characteristics of aggressive prostate cancer identified
For the first time ever, NTNU researchers have identified new characteristics of aggressive prostate cancer.
